7. Repeat prescribing self-assessment toolkit
7.1 Completing the repeat prescribing self-assessment tool
7.2 Suggested steps for GP practices/PCNs to complete the repeat prescribing self-assessment
7.3 Elements of repeat prescribing
7.4 RCGP/RPS repeat prescribing practice self-assessment
7.5 Community pharmacy and dispensing practices
7.6 RCGP/RPS practice/PCN repeat prescribing action plan template
Over the last 20 years, a considerable burden has been placed on general practices and community pharmacies as the demand, workload and complexity of repeat prescribing and dispensing has increased.
During this time, we have also seen safety issues emerge that are related to patients being able to access repeat medicines for years without always having the recommended checks and in-depth medication reviews taking place. This is especially relevant for opioids, antidepressants, benzodiazepines, gabapentinoids, antimicrobials, anticoagulants, lithium, methotrexate, azathioprine, leflunomide, diuretics, ACE inhibitors/A2RBs, ‘shared-care medicines’, teratogenic medicines for women of childbearing age and patients who take ten or more medicines. See higher risk medicines and scenarios described in Box 1.
This RCGP/RPS repeat prescribing self-assessment tool is aimed at helping each practice or PCN look critically at both the safety and efficiency of their repeat prescribing processes and agree as a practice team where to make improvements or address issues.
7.1 Completing the repeat prescribing self-assessment tool
The repeat prescribing self-assessment tool should ideally be discussed and completed in a practice or PCN meeting with both clinical and non-clinical staff present so that all perspectives can be heard. Where possible, local practice PPGs should also be involved. Everyone should be encouraged to be open about what is good about the current process, any gaps and where safety or efficiency in repeat prescribing can be improved and risks highlighted.
In addition, practice staff should speak to local community pharmacies to seek their input into areas where local arrangements cause problems or could be improved. Recent patient feedback (complaints or positive comments) should be highlighted during the discussions.
See questions for the general practice PPG in section 5.4.
There is a full appreciation of the current pressures in primary care. The first step for practices may be to discuss the self-assessment questions and be aware of issues or opportunities for improvement. If there are clear medication safety risks highlighted as part of the process, they should be addressed promptly.
However, for the majority of improvements, practices or PCNs may want to develop a repeat prescribing action plan (see section 7.6) which will be worked on over time by the whole practice team and external stakeholders, such as local community pharmacies and the PPG.
PCNs or practice pharmacists are well placed to lead this work, but improvements are most likely to happen where the whole practice team is involved in addressing any issues. Practice managers and prescribing lead GPs can play a key role in helping to keep up momentum and support any changes. This work will need a team/PCN approach.
There is wide variation in the way that repeat prescriptions are managed by individual practices. We appreciate that practices have very different capacity and capability challenges. To help with this, we have described ‘core’ questions for ALL practices to address and additional ‘advanced’ questions for practices/PCNs who wish to go further and ensure their repeat prescribing system is as robust as it can be.
7.2 Suggested steps for GP practices/PCNs to complete the repeat prescribing self-assessment
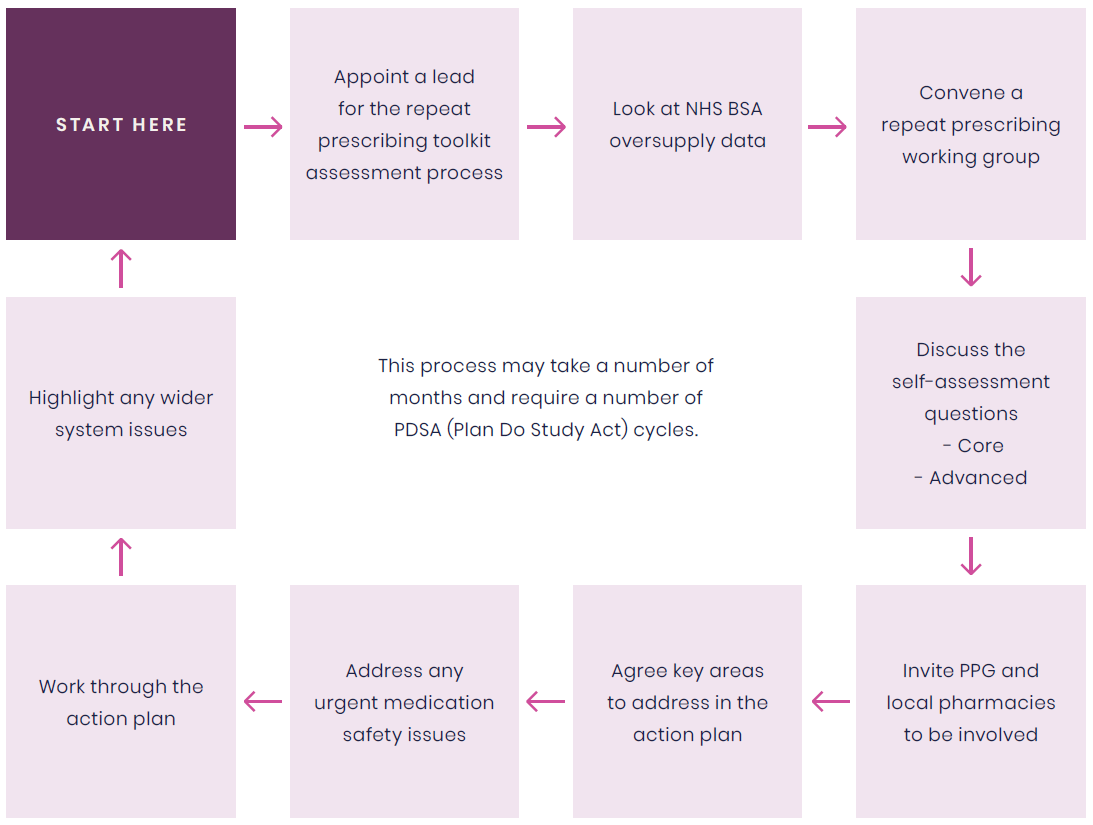
- Appoint a practice/PCN lead for the repeat prescribing self-assessment process
- Look at the oversupply dashboard hosted in ePACT2 (NHSBSA) to understand if there is an issue with oversupply in your practice
- Convene a ‘repeat prescribing working group’ to discuss the toolkit and self-assess the practice against the questions. Ideally, both clinical and non-clinical staff should be invited
- Review all responses
- Agree the key areas of action required and document these in the RCGP/RPS repeat prescribing action plan (see section 7.6)
- Prioritise any key medication safety issues highlighted during the process
- Work through the practice/PCN repeat prescribing action plan with SMART objectives assigned to members of the whole practice team and timelines agreed to keep the work on track
- Hold regular discussions to ensure actions are completed and new systems tested. See section 3 for process mapping and support to deliver quality improvement
- Where system-wide issues are affecting the safety or effectiveness of the repeat prescribing system, e.g., poor phlebotomy services causing lack of access to timely blood test monitoring, these should be formally highlighted to the ICB
- This process may take a number of months and require a number of PDSA (Plan Do Study Act) cycles.
Figure 4: Suggested steps for GP practices/PCNs to complete the repeat prescribing self-assessment
7.3 Elements of repeat prescribing
Research undertaken by Health Innovation East Midlands which used a ‘human factors’ perspective to understand opioid repeat prescribing, identified three crucial areas that have the potential to enhance the efficiency of repeat prescribing systems:
- Improve the functionality and use of the clinical systems
- Ensure there is an effective and embedded work procedure that is understood and accessible to all staff members and patients
- Create a manageable cognitive load/workload for clinical and non-clinical staff.27
These themes should be used to shape the discussions around the self-assessment.
The working group agreed that the repeat prescribing toolkit should centre around five key elements (see figure 3). These elements are important for the safe and effective running of a repeat prescribing system.
Practices will vary in their clinical, technical, and administrative capacity and capability. In the current workforce environment, it is not always possible to optimise all elements. However, by completing the self-assessment toolkit, practices will be able to identify current gaps and highlight where processes can be improved to ensure medication safety and maximise efficiency. This will require an open and honest approach from the practice and will need dedicated time to do well. Practices that have gone through such processes report that they have seen the benefits of this work, including clinician time saved, fewer prescription queries and faster turnaround (see examples in section 3).
The five elements of repeat prescribing:
1. Organisational culture
A just, open and positive organisational culture will ensure that the practice operates in an environment where issues related to the safety and efficiency of the repeat prescribing system can be raised, discussed, addressed and monitored for improvement.
This is important for medication safety but can be challenging to achieve. It relates to the culture within the practice but also the relationships with local community pharmacies and the PPG, as well as the registered patient population (see NHSE patient safety strategy).
Part of creating a positive organisational culture includes ensuring that the general practice has ownership of creating an effective process that improves the system performance, supporting staff to carry out their work well and delivers a service that is safe and timely for the patients needing it.
2. Patient/carer
Patients and carers have a role to play in the safe and efficient operating of a repeat prescribing process. The roles and responsibilities needed to optimise this process are described in the repeat prescribing patient partnership agreement (see the patient partnership agreement in section 5).
3. Clinical
This is where the clinical decision to authorise the repeat prescription is made. This element will determine for how long medicines are to be repeated and how regularly they are to be reviewed, as well as any monitoring requirements.
The quality and regularity of the medication review are key to the safety of this element. Practices will need to think about their staff skill mix to ensure that the right clinicians are engaged in the medication authorisation and review processes. GMC professional standards state that ‘clinicians prescribing repeat medications should only do so with adequate knowledge of the patient’s health and are satisfied that the drugs or treatment will meet their need’.28
Practices need to assess the quality of their medication reviews and if sufficient clinical input is allocated to patients receiving higher-risk medicines, in particular (see the glossary for authorisation/medication review and SMR definitions).
Patients prescribed higher risk medicines on repeat described in Box 1 should receive a regular, structured medication review to allow for their repeat medicines to be optimised and for any issues, concerns and expectations to be addressed. This will include any non-adherence, over or under ordering and any safety or monitoring issues.
Shared care arrangements
Good record keeping is an essential component of any clinical system and will ensure that all members of the team are sighted on the full clinical picture. Practices should ensure that where medicines are prescribed elsewhere, that clinical records are updated in a timely way. Guidance on how to do this is available from NHSE (Recording medicines prescribed elsewhere into the GP practice record).
Standardised shared care protocols are also available from NHSE.
4. Technical
Technical processes should be in place BEFORE a repeat medication is reauthorised to ensure that routine monitoring and follow up actions are highlighted and completed. Use digital systems to optimise the repeat prescribing process.
The technical element of the repeat prescribing system should include alerts to prescribers to highlight under or over ordering and should ensure that medicines that have been deprescribed cannot inadvertently be restarted without a clinician’s input. Technical optimisation, with respect to repeat prescribing, will ensure that all digital and technical systems are deployed and precious clinical and administrative staff are not carrying out routine tasks that can be safely automated (see section 8.2 for SystmOne and EMIS examples).
The technical element of repeat prescribing also highlights the opportunity that wider deployment of technical staff, which may include pharmacy technicians, can offer to ensure that clinical staff resources are used sensibly.
5. Administrative
Administrative staff play a significant role in the day-to-day operation of the repeat prescribing system. They are at the forefront of managing queries and problems and often have oversight of the whole process. They need time for training in how to manage repeat prescriptions safely as well as a thorough induction of how the practice operates the process. They need to work closely with community pharmacy colleagues.
Support and training are not provided to all administrative staff working on repeat medication and yet this is a high-risk component of their role as well as a high-risk element of the process for the practice.
7.4 RCGP/RPS repeat prescribing practice self-assessment
Organisational culture
CORE
- Is there a written policy/standard operating procedure (SOP) to describe the planned process for repeat prescribing and medication reviews in the practice (is it fit for purpose, easy to access and easy to use)?
- Is there training for all staff involved in the repeat prescribing process (administrative and clinical) to ensure they are aware of the policy/SOP and understand the practice/PCN repeat prescribing process? What about locum staff?
- Who in the practice has overall responsibility for the repeat prescribing process?
- Who in the practice has responsibility for the day-to-day running of the repeat prescribing process?
- Is it clear how and who in the practice will deal with an issue, incident or complaint relating to repeat prescriptions?
- Are practice staff clear about the roles and responsibilities of all staff members involved in the repeat prescribing process?
- If any member of staff has a concern about the repeat prescribing process, is it clear how to raise issues and offer solutions, and is that effective?
- Is the repeat prescribing process and how it works (including time associated within the practice and the community pharmacy), clearly communicated to patients and are all patients clear about how they are to engage with it?
- What is the mechanism for the practice to discuss how the current system is working?
- In your discussions to review the repeat prescribing system are the following included:
- The input/views of local pharmacies and or the PCN community pharmacy lead?
- How high-risk medicines and high-risk scenarios are managed and if this is working effectively?
- Any recent incidents or complaints and what can be learned for them?
- How you measure if the current system is working? This should include the NHSBSA oversupply data and any local metrics on time taken for repeats.
- Is dedicated time allocated to clinical and non-clinical staff to manage repeats?
ADVANCED
- Is the repeat prescribing process treated as a risk activity, e.g., is it treated as a separate task, is dedicated room provided for administrative staff processing repeats? Is time given to clinical staff deal with authorisation?
- How well does the practice liaise with local community pharmacies to resolve any frequently occurring issues?
- How has the practice learned from a recent repeat medication incident or complaint?
- How has the practice process mapped and reviewed the workload associated with repeats? Was this for both clinical and non-clinical staff?
- How is the practice using digital solutions to reduce workload? What else could be done? Is GP clinical system functionality optimised by all staff and has the practice explored the use of eRD and the NHS app?
- Are SMRs embedded in the culture of the practice and given the space and time required to undertake them? Are patients at higher risk of medicines-related harm prioritised for an SMR?
- Is the burden of the repeat prescribing system on clinical staff regularly reviewed and revised if individuals are experiencing unsafe workloads? How is this assessed? Does the practice regularly collect and review data relating to numbers of requests dealt with by admin/pharmacy team/GPs?
- Does the practice have a member of staff who can support patients who struggle to use the usual repeat prescribing process such as those with learning difficulties, those unable to use digital processes and those where a chaotic lifestyle can make it harder to order and collect prescriptions, such as those with no fixed home address?
Clinical responsibilities
CORE
- When a medicine is moved from acute to repeat, is this decision explicitly recorded with a duration of the repeat documented?
- Is the indication for a repeat medication clearly documented in the GP clinical system?
- How are patients informed about any risks of longer-term use of the medicine(s) and the expected length of the prescription at initiation? Is this clearly documented in the notes?
- Is there a separate process for higher risk repeat medicines? (see box 1)
- How are the responsibilities around repeat medicines communicated to patients (e.g., monitoring requirements, medication reviews and when medicines will not be repeated)?
- How can community pharmacies speak to a member of the clinical practice team to ask questions about a repeat prescription from a clinical or safety perspective? Is this working well?
- What systems are in place to monitor over ordering of repeat medicines (especially in relation to medicines with dependence-forming or overdose potential)?
- What processes are in place to identify and manage under ordering of repeat medicines?
- How are re-authorisations/medication reviews/SMRs (see definitions on page 16) incorporated into the repeat prescribing process and what happens if the patient does not participate?
- When medicines are recommended by secondary care, is there a clear and safe clinical authorisation process for discharge letters to be interpreted and medicines clinically authorised for repeat?
- When medicines are stopped, is there a clear process to ensure they are removed from the repeat medicines list?
- Is there a clear process of action for the Practice to respond to national medication alerts such as national patient safety alerts or drug safety alerts that relate to medicines prescribed on repeat?
ADVANCED
- Are terms such as ‘as directed’ or ‘when required’ avoided, especially when in relation to high-risk medicines? How is feedback provided to prescribers when clear directions are not provided?
- Is there a clear process for dealing with prescription queries?
- How are prescription queries monitored? Many prescription queries might indicate a process problem. The same patients querying each cycle might indicate they are unsure of the correct process. How are both scenarios addressed?
- Have there been any serious incidents involving repeat medicines and how were lessons learned?
Technical
CORE
- What is the process for requests for repeat prescriptions? How many ways can patients request repeats? Is this manageable for the practice? Is it safe and clear to patients?
- How are repeat prescription requests triaged?
- Is there a clear medicine resupply practice policy for support staff, e.g., it is within the medication review date documented on the system or it has a specified number of authorisations that are still within a valid time period?
- How are checks made around monitoring requirements, such as blood tests?
- How does the practice ensure monitoring requirements are adhered to before repeat prescriptions are re-authorised?
- What is the practice’s process if a patient does not engage with monitoring arrangements? How is this communicated to patients?
- How are large scale requests managed (e.g., care homes)? Is this working well?
- How are dose changes that are needed mid prescription-authorisation cycle made, so that the patient receives the new dose, and the clinical notes are updated to prevent older dosing schedules being prescribed inadvertently?
- How does the practice identify and manage over-ordering of medicines (especially those with
dependence-forming or overdose potential?)
- How does the practice identify and monitor under ordering?
- How does the practice manage discharge letters involving requests for repeat prescriptions? How are discharge issues flagged to clinicians?
- How are ‘shared care’ arrangements for prescriptions managed safely? How is this documented so that all clinicians are aware of the full clinical picture?
- Could the practice improve efficiency in how patients request repeats and receive medication requests?
ADVANCED
- What (digital) functions of the clinical system are used to help with the efficiency of the practice repeat prescribing system? Do all staff consistently apply these functions?
- Is there additional system functionality that is not deployed and why?
- What is the agreed process for interactions between the community pharmacy and the practice?
- Is there a technical lead for repeat prescriptions who is responsible for monitoring workload, queries, system failures, inefficiencies and safety issues?
- Does the practice regularly monitor the number of repeat requests per month and audit how the workload associated with the volume of requests falls to various team members (clinical and non-clinical)?
- Does the practice monitor how many repeat prescription requests are made via 111? Is this excessive? ICB-level NHS 111 data is updated monthly. Could high usage of 111 indicate that there is a problem with your repeat prescribing process?
Administrative
CORE
- Who in the practice manages the day-to-day administrative duties of the repeat prescription processes?
- Is there a very clear process where all relevant members of the administrative team understand their roles and their limits of what they are authorised to do?
- How are administrative staff trained?
- How are administrative staff supported in their repeat prescription roles?
- Is there a process for dealing with queries that do not fit the usual process, and is this clear to administrative staff (e.g., ordered too early, essential blood tests not available, patient hasn’t had the medicine for a number of months or over ordering)?
- Are administrative staff aware of the risks of circumventing the agreed practice process to expedite
prescription queries?
ADVANCED
- Are the administrative team aware of which medicines are higher risk repeat medicines, how they are to be managed and how this might differ from the usual repeat process?
- Are administrative staff given a quiet space to process repeat prescriptions with minimal interruptions?
- Is there a dedicated phoneline/extension for community pharmacies to contact the practice team in relation to prescription queries?
- Does the practice regularly monitor the volume and nature of repeat ‘queries’, i.e., where the request or query does not follow the usual process agreed for repeats?
See patient questions in section 5.4.
7.5 Community pharmacy and dispensing practices
Community pharmacies and their local general practices need to work closely together to ensure the safe and efficient running of the repeat prescribing pathway. Both services have come under increasing pressure in recent years but should still aim to engage regularly to ensure medicines are managed effectively for patients.
Pharmacies and general practices/PCNs should aim to meet to discuss this repeat prescribing self-assessment toolkit and how any local issues can be resolved or improved. Some PCNs will have a dedicated community pharmacy lead who can support this.
Both parts of the pathway (prescribing and dispensing) are complex and hugely challenging. Both partners need to recognise the constraints experienced by the other to try to ensure that patients are provided with a safe and efficient local process.
Key questions for community pharmacy teams to assess their repeat prescription provision:
- Is your process for receiving and dispensing repeat prescriptions clear to your patients and local general practices?
- Is the time needed for the safe dispensing of repeat medication communicated to patients, so they are aware how far in advance to order their next supply?
- Does your repeat prescribing process work equally well for all prescriptions from all local general practices?
- How do you communicate urgent queries to the GP practice, and is there an audit trail in place for this?
- How do you communicate non-urgent queries to the GP practice? Is this process effective? How do you audit this?
- Have you worked with local GP practices to encourage the use of eRD and support implementation ,for patients who meet the criteria for this?
- What role does the pharmacy play in ordering repeat medication for patients? Has this been agreed with local GP practices?
- Is there a process for highlighting under/over ordering of medication to the GP practice?
- How do you encourage patients to use digital solutions, such as the NHS app, to order and check on repeat prescriptions?
- Do you have clear process of action to respond to national alerts such as national patient safety alerts of drug safety updates?
Dispensing practices
General practices in more rural areas offering a dispensing service should consider the following questions:
- Are there SOPs in place within the dispensary covering the processes for repeat prescribing covering roles, responsibilities and training of staff?
- Is your process for receiving and dispensing repeat prescriptions clear to your patients?
- Is the time for dispensing of repeat medication communicated to patients so they are aware how far in advance to order their next supply?
- Do you encourage patients to use digital solutions such as the NHS app to order and check on repeat prescriptions?
- What support and training are in place for dispensary staff who operate the repeat prescribing system?
- Are all repeat prescriptions signed by the prescribing clinician before they are dispensed?
7.6 RCGP/RPS practice/PCN repeat prescribing action plan template
System issues to highlight to the ICB
Pathways issue to discuss with local pharmacies