By Ciara Duffy, RPS England Pharmacy Board and Assembly member

The UK is on the cusp of a major transformation in pharmaceutical manufacturing with the introduction of Decentralised Manufacturing (DM), which came into effect on 23 July 2025 under Statutory Instrument 2025 No.87. This regulatory framework amends The Human Medicines Regulations 201(2) and The Medicines for Human Use (Clinical Trials) Regulations 2004(3), allowing the production of medicinal products at or near the point of care rather than in traditional, centralised facilities. DM represents a patient-centric shift designed to enhance supply chain resilience, promote technological innovation, and deliver medicines closer to the patient, where speed and responsiveness matter most. It is important to highlight that this new legislation is designed to facilitate the provision of therapies where technological and regulatory barriers previously prevented their delivery, rather than to replace or override conventional manufacturing regulations or processes.
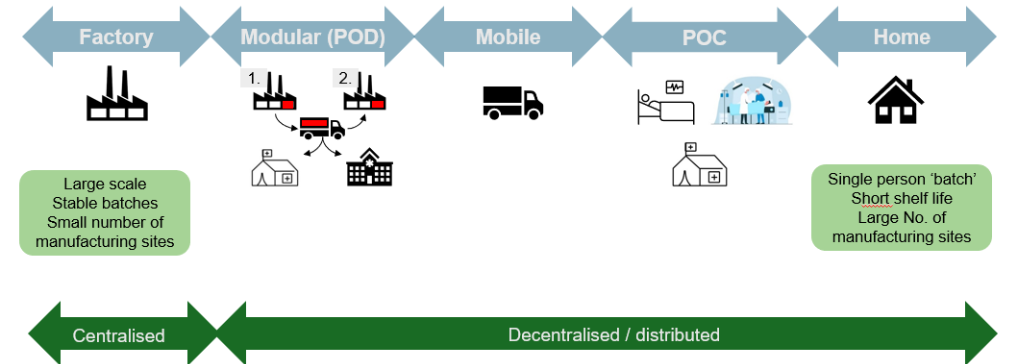
Figure 1: Broadened Spectrum of Manufacture – MHRA Webinar Oct 2024.
Hub and Spoke
DM introduces a hub-and-spoke model, where a licensed Control Site serves as the central hub, responsible for oversight, regulatory compliance and maintaining the Decentralised Manufacturing Master File (DMMF)(4). Multiple remote manufacturing units (spokes) , such as hospitals, modular mobile units, community pharmacies or approved home environments, handle specific manufacturing steps closer to the point of delivery. This structure enables more flexible and adaptive supply chains, particularly suited to therapies like Advanced Therapy Medicinal Products (ATMPs), vaccines, and on-demand biologics.
- A single Control Site (hub) holds the manufacturing licence and oversees quality, regulatory compliance, and the Decentralised Manufacture Master File (DMMF).
- Multiple remote manufacturing units (spokes), which may include hospital-based facilities, modular mobile units, or even controlled home environments, execute specific manufacturing steps.
Legal tests and the DM designation-definitions
Decentralised Manufacturing is permitted only when the product meets one of two legal tests established by the MHRA:
Point-of-Care (POC) manufacturing
A product qualifies where, for reasons relating to method of manufacture, shelf life, constituents, or method or route of administration, it can only be manufactured at or near the point of use or administration(1). Convenience or cost is not an acceptable justification(5). POC manufacture can occur via: 1. mobile manufacture in a 2. healthcare establishment or 3. home of patient(6).
Modular Manufacture (MM)
A product qualifies where, for reasons relating to deployment, it is deemed necessary or expedient by the licensing authority to be manufactured or assembled in a modular unit(1), typically to address public health needs or deliver significant clinical advantage.
Applicants must submit justification with supporting data and designation must be confirmed(5) before submitting a Marketing Authorisation(MA), Clinical Trial authorisation (CTA), or manufacturing licence application which can be a Manufacture Import Authorisation (MIA), a MIA(Investigational Medicinal Products) (MIA(IMP)) or a Manufacturers Specials (MS) authorisation.
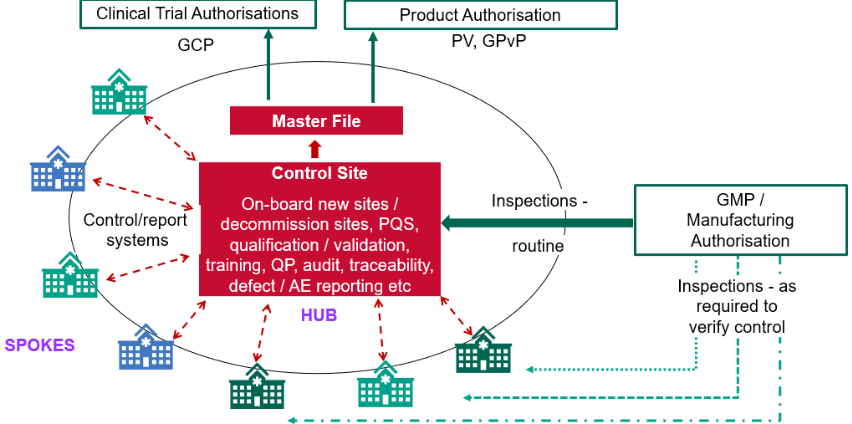
Figure 2: Decentralised Manufacture Oversight – MHRA Webinar Oct 2024.
GMP oversight and the role of the qualified person
Despite the legislative changes, the fundamental requirements which assure safety, quality and efficacy remain unchanged, these new elements are integrated and built onto existing GMP frameworks, ensuring all standard obligations continue to apply. Under decentralised manufacture, the Control Site is legally responsible for ensuring GMP compliance across all remote manufacturing units. This oversight includes site qualification, equipment validation, operator training and maintaining data integrity and traceability.
The Qualified Person remains responsible for the release of all IMP or commercial DM products including post-administration when necessary(7). Their role ensures that decentralised operations deliver equivalent control and quality outcomes to traditional centralised processes.
Governance in healthcare and the role of pharmacy teams
Pharmacists and pharmacy teams are poised to play a vital role in decentralised manufacturing, particularly within healthcare settings such as NHS hospitals and community-based environments. Learning from the introduction of other disruptive medicines, such as ATMPs, will be invaluable. However, it is important to recognise that the risks associated with product manufacture in clinical settings are distinct and represent a significant step change from traditional preparation activities.
Additionally, the responsibilities of dispensary, ward-based, and community pharmacy teams are likely to expand beyond traditional product handling to encompass activities such as:
- Equipment checks and calibration;
- Local release of products;
- Documentation of GMP processes;
- Identifying deviations and ensuring corrective actions.
This expanded scope of practice will necessitate formal collaboration between pharmacy teams and Control Sites, along with ongoing GMP training and the adoption of governance frameworks that integrate manufacturing responsibilities smoothly and effectively. Fortunately, NHS technical service teams already possess extensive GMP expertise and are well-equipped to provide invaluable support to their pharmacy colleagues in navigating these new responsibilities.
Section 10 exemption: Understand the limits
It is important to clarify that the Section 10 exemption refers exclusively to preparation(8), not manufacture. As a result, decentralised manufacturing is excluded from the Section 10 exemption under the Medicines Act 1968. Extemporaneously prepared medicines under Section 10 are limited to named patient use only and are not suitable for complex, high-risk, or biological products. Pharmacists must adhere to GPhC guidance, ensure medicines are used appropriately and seek early advice from NHS QA Specialists if any activity resembling decentralised manufacturing is contemplated under Section 10.
Global regulatory alignment
The UK’s leadership in decentralised manufacturing is mirrored by global regulatory efforts to adopt to innovate manufacturing methods:
The International Coalition of Medicines Regulatory Authorities (ICMRA) convened a 2024 workshop(9) to harmonise definitions and terminology, explore GxP oversight models and share strategies for process comparability, site inspection, and digital control.
The European Medicines Agency (EMA), via the Quality Innovation Group, is drafting legislation to permit multiple POC sites under a single manufacturing authorisation and focus on short shelf-life ATMPs requiring localised manufacture and/or testing(10).
The FDA supports decentralised manufacture through publication of a 2022 discussion paper defining centralised oversight of decentralised systems(11),a draft 2025 guidance clarifying how manufacturers can comply with 21 CFR 211.110, using real-time monitoring, hybrid in-process controls and digital data models to assure batch quality using advanced manufacturing methods(12).
These global efforts reflect a shared ambition to enable flexible manufacturing models without compromising quality or regulatory assurance.
Conclusion
Decentralised Manufacturing offers a transformative opportunity to supply medicines closer to the patient, flexibly, safely and with regulatory clarity. However, it also brings complexity, requiring shared responsibility, training and alignment across all parts of the system. Success depends on:
- Regulatory readiness – clear legal designation and licensing authorisations.
- Technical readiness – validated, repeatable, GMP-compliant, traceable processes across multiple locations.
- Institutional readiness – strong governance, collaboration, and professional accountability.
This pioneering framework also reflects the MHRA’s commitment to forward-thinking regulation, changing legislation not only to be innovative, enduring and enabling but also responsive and centred on patient need at every stage of the medicines lifecycle.
This evolving model requires robust cross-sector collaboration involving industry, the NHS, private providers, regulators and academia. It also presents a significant opportunity for pharmacy professionals to take a leading role in driving innovation, strengthening governance and upholding the highest standards of patient safety, quality and efficacy. To support this transition, the MHRA has developed a comprehensive suite of guidance documents and webinars, which are accessible via the provided links.
References:
- The Human Medicines (Amendment) (Modular Manufacture and Point of Care) Regulations 2025
- The Human Medicines Regulations 2012
- The Medicines for Human Use (Clinical Trials) Regulations 2004
- Human medicines Modular Manufacture and Point of Care regulations 2025: Overview - GOV.UK
- Decentralised Manufacture: The designation step - GOV.UK
- Decentralised manufacture hub - GOV.UK
- Decentralised manufacture: UK Guideline on Good Manufacturing Practice (GMP) - GOV.UK
- Medicines Act 1968
- ICMRA Workshop on Decentralised or Distributed Manufacturing | International Coalition of Medicines Regulatory Authorities (ICMRA)
- QIG workplan 2025-2027
- Federal Register :: Discussion Paper: Distributed Manufacturing and Point-of-Care Manufacturing of Drugs; Request for Information and Comments
- Considerations for Complying With 21 CFR 211.110